Pyridam Farma Distributes Osteoporosis Medication from EffRx Pharmaceuticals Swiss
Jakarta, May 2, 2024 – EffRx Pharmaceuticals SA, a Swiss pharmaceutical company focused on developing and commercializing musculoskeletal and rare disease drugs, today announced that it has signed an exclusive License and Distribution Agreement for Binosto® in Indonesia with Pyridam Farma.
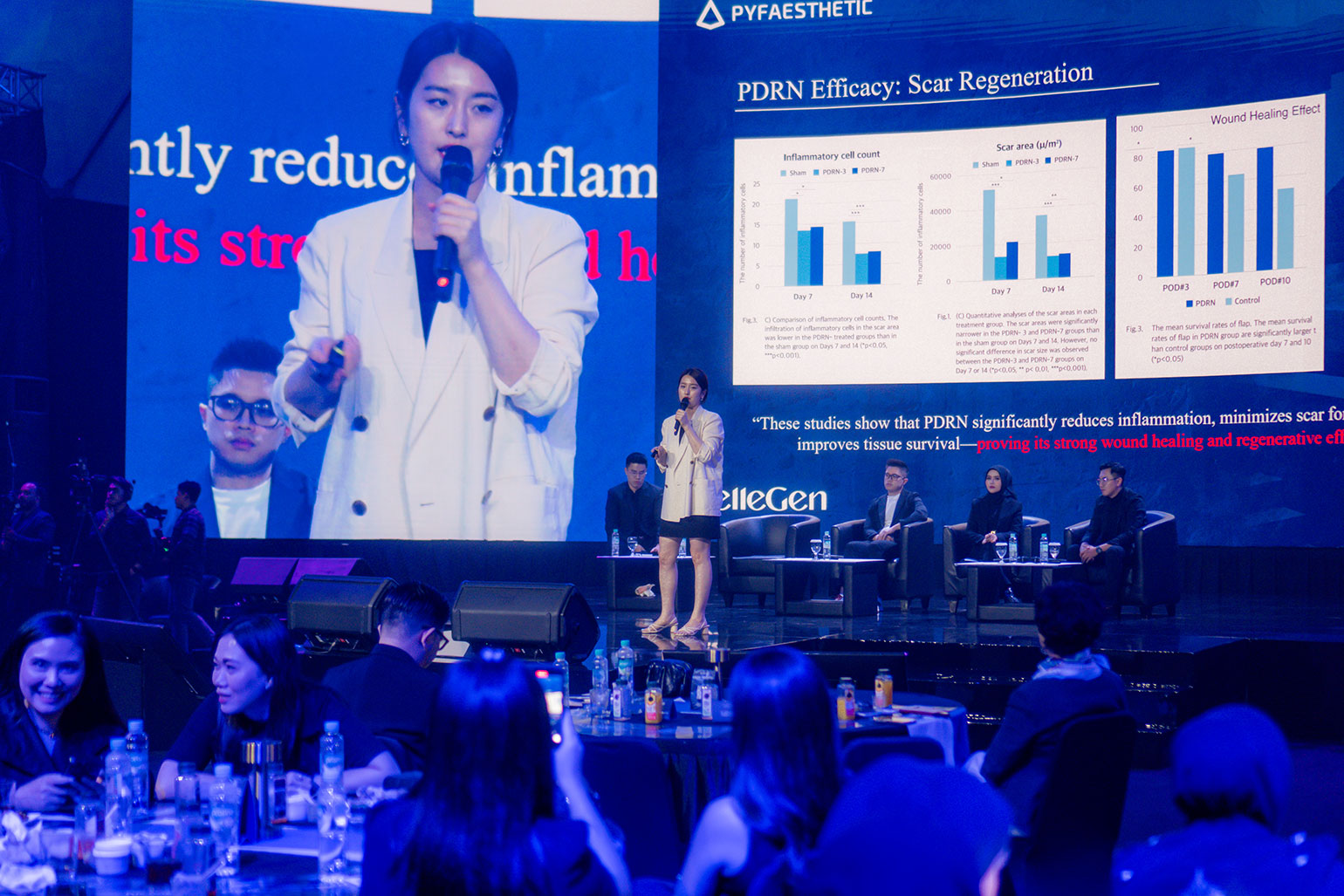
Binosto® (buffered alendronate) is indicated for the treatment of postmenopausal osteoporosis and is currently marketed in Europe, the US, the Middle East, and Asia. Binosto® offers an important treatment option for postmenopausal women with osteoporosis, and effectively reduces the risk of fractures. Binosto® is associated with a lower frequency of upper gastrointestinal side effects and demonstrates higher persistence compared to reported cases with alendronate tablets.
Widjanarko Brotosaputro, Director of Pyridam Farma said, “We are very pleased to be able to collaborate with EffRx Pharmaceuticals and are very much looking forward to the positive impact on the accessibility of healthcare services in Indonesia. We believe that this collaboration has the potential to significantly increase access to healthcare services for osteoporosis patients and provide innovative solutions to the national healthcare system. Together we are committed to driving positive change and improving patient health in Indonesia.”
Lorenzo Bosisio, CEO of EffRx, said, “We are delighted to announce our partnership with Pyridam Farma to further expand Binosto®’s footprint. Pyridam Farma is a dynamic and recognized healthcare company with strong roots in the Indonesian market, making it the partner of choice for our company. Pyridam’s commitment to patients living healthier lives aligns with Binosto®’s promise to alleviate the burden of osteoporosis for Indonesian women.”
Under the terms of the agreement, EffRx will grant Pyridam Farma an exclusive license to commercialize Binosto® in Indonesia and will also supply the product.
About Binosto®:
Binosto® (alendronate effervescent tablet 70 mg) is the first and only effervescent alendronate tablet with a buffered solution*, for the treatment of postmenopausal osteoporosis and to increase bone mass in men. Binosto® can reduce the risk of hip, spine, and non-spine fractures. Binosto® is taken once a week as an easy-to-swallow buffered solution without the risk of the tablet getting stuck in the esophagus. The buffered solution with high acid neutralizing capacity minimizes the risk of esophageal and gastric exposure to acidified alendronate and has a higher persistence compared to tablet form, reducing the risk of fractures in osteoporosis patients.
*solution that does not change its pH significantly on dilution or if acid or base is added at a constant temperature
Disclaimer: Please refer to the official Binosto® prescribing information and approved indications, contraindications, and warnings in your country.
About EffRx Pharmaceuticals:
EffRx Pharmaceuticals is a Swiss pharmaceutical company focused on developing and commercializing musculoskeletal and rare disease drugs. EffRx’s main commercial product, Binosto® for the treatment of osteoporosis, is successfully commercialized by Licensees in European, Asian and Middle Eastern countries. EffRx is controlled by Abiogen Pharma S.p.A., a leading Italian company in the field of osteoarticular diseases and bone metabolism.
About PT Pyridam Farma Tbk.
PT Pyridam Farma Tbk is a pharmaceutical company with its main business in the industry and commercialization of modern and traditional medicines and the distribution of medical devices such as laboratory equipment and also PCR test kits. The company was founded in 1976 and has been a public company listed on the Indonesia Stock Exchange since 2001.
PT Pyridam Farma Tbk. produces a wide variety of pharmaceutical products ranging from Antibiotics, Vitamins, Health Supplements, to Traditional Medicines. The company has more than 200 products in the form of effervescent tablets, caplets, capsules, syrups, and creams.
PT Pyridam Farma Tbk also provides toll manufacturing services and MA Holders and has partnered with more than 10 global pharmaceutical companies to provide registration services along with product marketing.
For more information, contact:
Pyridam Farma Kezia Mareshah Corporate Communication Manager Pyridam Farma (PYFA) Email: corcomm@pyfa.co.id Phone number: 08118010391
EffRx Media info@effrx.com