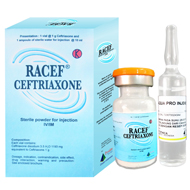
Description
COMPOSITION
Each vial contains : sterile Ceftriaxone Disodium 3.5 H2O 1.193 g equivalent to 1.0 g
PHARMACOLOGY
Ceftriaxone is an antimicrobial from cephalosporin group which has a broad spectrum against Gram positive and Gram
negative bacteria, including anaerobic bacteria with plasma elimination half life of about 8 hours. Ceftriaxone is stable against
beta lactamase (either Penicillinase or Cephalosporinase) from Gram positive and Gram negative bacteria. Ceftriaxone exerts
bactericidal effect through the inhibition of bacterial cell wall synthesis. Ceftriaxone’s half life is 8 hours, 60% of it is excreted
in active form through urine and the rest is excreted through feces.
Pharmacokinetic : the use of ceftriaxone in the elderly and patients with renal disorder is only slight different from that in
healthy adult patients. Therefore, the dose of ceftriaxone up to 2 g daily is not necessarily adjusted in those patients.
Antibacterial spectrum of ceftriaxone include most of Gram positive and Gram negative bacteria either aerobic or anaerobic
which is clinically significant is as follows:
Aerobic Gram positive: Staphylococcus aureus (including penicillinase-producing strains), Staphylococcus epidermidis,
Streptococcus pneumoniae, Streptococcus pyogenes, Viridans group Streptococci, Streptococcus agalactiae.
Aerobic Gram negative: Acinetobacter calcoaceticus, Enterobacter aerogenes, Enterobacter cloacae, E. coli, Haemophylus
influenzae (including ampicillin-resistant strains and Betalactamase-producing strains), Haemophilus parainfluenzae,
Klebsialle oxytoca, Klebsiaella pneumoniae, Moraxella catarrhalis (beta lactamase-producing strains), Morganella
morganii, Neisseria gonorrhoeae (including Penicillinase and non-penicillinase – producing strains), Neisseria
meningitidis, Proteus mirabilis, Proteus vulgaris, Srratia marcescens, Pseudomonas aeruginosa, Citrobacter diversus,
Citrobacter freundii, Providencia sp. (including Providencia rettgeri), Salmonella sp. (including Salmonella typhi),
shigella sp.
Anaerobic bacteria : Clostridium sp., Peptostreptococcus sp., Bacteroides bivius.
INDICATIONS
Infections caused by microorganisms susceptible to ceftriaxone such as :
Prophylaxis of pre-operative infection
Infections of bone, skin and soft tissues
Infections which damage body defense mechanism
Abdominal infections (peritonitis, tissue and gastrointestinal infections)
Urinary tract and renal infections
Respiratory tract infections particularly pneumonia, ear, nose and throat infections.
Genital infections including gonorrhoea
Sepsis meningitis
CONTRA INDICATIONS
Patients hypersensitive to cephalosporins. In patients with hypersensitivity to penicillin, the possibility of cross-reaction should
be considered.
DOSAGES
Meningitis treatment in infants and children should be started with 100 mg/kg BW (do not exceed 4000 mg)
Usual dosages :
Adults and children > 12 years: usually 1-2 g once daily (every 24 hours). In several cases or in infections caused by
susceptible organisms, dosage may be increased to 4 g daily.
Neonates and infants and children < 12 years : as recommended for the use of once daily.
Neonates (age < 14 days) : 20-50 mg/kgBW/day (maximum 50 mg/kg BW/day)
Infants and children 15 days – 12 years : 20-80 mg/kgBW/day. For children with body weight > 50 kg dosage for adult
is recommended.
I.V dose of 50 mg or more (per kg BW) should be given through infusion for at least 30 minutes.
Elderly patients: should be the same as the dosage for adults (no dosage adjustment is required).
The duration of treatment may vary according to the severity of the disease. As with other antibiotics, the use of
ceftriaxone should be continuously minimum 48/72 hours after the patients had fever or after the result of the bacterial
eradication is acquired.
Special dosage :
Meningitis: for the treatment of bacterial meningitis in infants and children the initial dose given is 100 mg/kg BW
(maximum 1 g) once daily. After the causative organism is identified and determined then the dose may be reduced.
The following duration of treatment has shown its effectiveness in bacteria :
Neisseria meningitidis : 4 days
Haemophilus influenzae : 6 days
Steptococcus pneumoniae : 7 days
Gonorrhoeae: for the treatment of gonorrhoeae (Penicillinase and non-penicillinase-producing strains) single
dose of 250 mg i.m is recommended.
The use in pre-surgery: for the prophylaxis of post-operative infection (or potentially contaminated during the surgery)
it is recommended to adjust the dosage according to the risk level of infection. A single dose of 1 – 2 g of ceftriaxone
should be administered 30 – 90 minutes before colorectal surgery the administration of ceftriaxone (though separately)
with or without 5-Nitro-imidazole (e.g. Omidazole) has been proven effective.
Patients with renal and liver function impairment : no dosage adjustment required if the liver function is good, except in
the case of preterminal renal impairment (creatinine clearance < 10 ml/minutes) the daily dose should be limited
maximum 2 g/day. It is not recommended to be given in patients with liver and renal function impairment.
Patients under dialysis treatment: no additional supplementary dose following dialysis guide. However, the serum
concentration must be monitored to decide whether dose adjustment is required when the elimination rate might be
reduced.
I.M administration: ceftriaxone is reconstituted with an appropriate solvent. After reconstitution, each ml of solution contains
250 – 350 mg of ceftriaxone (depending on the amount of solvent added). The amount of solvent may be added when
necessary.
Vial Amount of solvent added
250 mg/mL 350 mg/mL
1 g 3.6 mL 2.1 mL
I.V administration : ceftriaxone 1 g is dissolved in 10 ml sterile water for injection, afterwards it should be given in bolus i.v.
within 2 – 4 minutes. The reconstituted solution is stable in 2 days at room temperature.
SIDE EFFECTS
Local : pain at the injection site, induration (reaction of pain after i.v. administration)
Hypersensitivity reaction : rash dan pruritus
Hematology : eosinofilia, leucopenia hemolytic and thrombocytosis
Gastrointestinal tract : nausea, vomiting, diarrhea and dysgesia
Hepar : increased SGOT, SGPT, Alkalin fosfatase and bilirubin
Renal : increased BUN value
CNS : dizziness, headache
Genitourinary : vaginitis and moniliasis
Others : sweat, diaforesis, leucocytosis, limphocytosis, monocytosis, basophilia, reduced prothrombin time, jaundice,
gallbladder sludge, glycosuria, hematuria, anaphylaxis, bronchospasm, serum sickness, abdominal pain, colitis,
flatulence, dyspepsia, epistaxis, biliary utiasis, agranulocytosis, renal precipitation and nefrolitiasis.
Concomitant use of ceftriaxone and calcium may induce precipitation in lugs and renal even causing death in infants /
neonates.
WARNING AND PRECAUTIONS
Before treatment with ceftriaxone, hypersensitivity test against penicillin and cephalosporin should be done.
As with other cephalosporin antibiotics, anaphylactic shock can not be avoided though anamnesis has been conducted.
Anaphylactic shock needs immediate treatment such as epinephrine (adrenaline) IV followed by glucocorticoid. In vitro
researches showed that ceftriaxone as with other cephalosporins replaces bilirubin binding with serum albumin.
Ceftriaxone should not be given to infants/neonates with hyperbilirubinemia (especially premature babies).
During prolonged use, blood examination should be conducted regularly. Ceftiraxone use may cause growth of several non-
susceptible microorganisms against ceftriaxone particularly Candida, Enterococci, Bacteroides fragilis, Pseudomonas
aeruginosa. In the case of superinfection or suprainfection, give appropriate treatment.
As with cephalosporin antibiotics, ceftriaxone influences blood glucose test which uses cupri sulfate (Benedict’s, Clinitest) but
not in the use of glucose oxidase method (Clinistix, Test tape).
Pseudomembranous colitis has ever been reported due to the use of most of antibiotics, including ceftriaxone, therefore
diagnosis in patients with diarrhea due to antibiotic should be taken into consideration.
Ceftriaxone is excreted through bile and renal. Thus, the serum concentration of ceftriaxone should be done in patients with
renal function impairment. In case of accumulation dosage reduction should be considered.
No dosage adjustment required in patients with liver function disorder, except in patients with severe renal and liver function
impairment, the dose should not exceed 2 g daily without monitoring the serum concentration.
It should be used cautiously in patients with a history of gastrointestinal disease esp. colitis. The use of this drug in patients
with vitamin K synthesis disorder or low intake of vitamin K (such as patients with chronic liver disease and malnutrition) thus
prothrombin time should be monitored.
Prolonged use may induce growth of non-susceptible microorganisms. In case of superinfection, discontinue use and replace
with appropriate therapy.
Pseudomembranous colitis has been reported due to the use of antibiotics including ceftriaxone. Thus the use of ceftriaxone
should be reconsidered if diarrhea occurs.
The use of ceftriaxone in pregnant women is allowed only if necessary.
Do not give ceftriaxone in breastfeeding women because ceftriaxone is excreted in breast milk.
PHARMACEUTICAL PRECAUTIONS
Ceftriaxone should not be mixed in spuit with aminoglicoside.
DRUG INTERACTIONS
Combinations with the following drugs :
Anticoagulant : may increase anticoagulant effects of warfarin.
Aminoglicoside: in vitro researches indicated that antibacterial activity of ceftriaxone and aminoglicoside (amikacin,
gentamicin dan tobramycin) is additive or synergistic against several Enterobacter strains and several Pseudomonas
aeruginosa strains.
Loop-diuretic drugs may increase risk of nephrotoxicity.
Uricosuric – Probenecid may reduce ceftriaxone excretion
Vancomycin and fluconazole: both drugs are incompatible physically with ceftriaxone.
PRESENTATION
Box, 1 vial @ 1 g sterile dry powder of cefriaxone and 1 ampoule of sterile water for injection @ 10 ml.
On medical prescription only.
Store at room temperature (25 – 30 ◦C), protected from light.